Sqf 13 Prerequisite Programs
FSSC 22000 requires “Prerequisite Programs, (PRPs)”. These are programs and practices put in place to ensure that the environment is clean, sanitary and appropriate for manufacturing safe product. PRPs include the programs that many food processors call GMPs. FSM eDigest October 3, 2017 What to Expect from SQF Modifications. By Susan Moyers, Ph.D., M.P.H., and Allen Sayler. While 2017 is a year for manufacturing. SQF ISO FSSC GMP Programs for Food Safety Management Systems Certification GMPs and FSSC 22000 FSSC 22000 requires “Prerequisite Programs, (PRPs)”. •These are programs and practices put in place to ensure that the environment is clean, sanitary and appropriate for manufacturing safe product. •PRPs include. Prerequisite Programs for GMPs. Free Download Achi Ir Pro Sc Manual Programs Like Photoshop. Prerequisite programs are procedures, including. GMPs, that address operational conditions providing the foundation for HACCP. Certain programs and activities are required and must be in place if a HACCP program is to be effective. In this chapter we introduce these prerequisite.
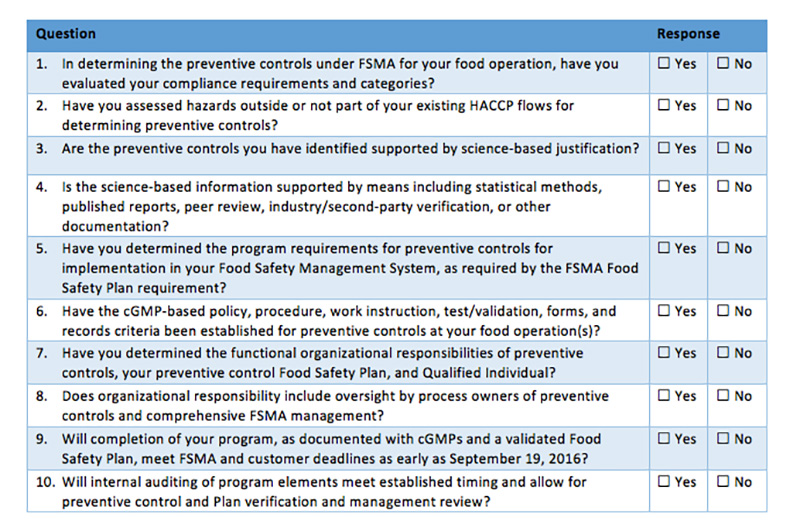
SQF 4.5.2 requires the validation of Pre-requisite programs and records of all validation activities. Clause 4.5.2.1 The methods, responsibility and criteria for validating Pre-requisite Programs and critical food safety limits to ensure they achieve their intended purpose shall be documented and implemented. Clause 4.5.2.2 Records of all validation activities shall be maintained.

Beckman Coulter N5 Manually. Can one of you experts provide examples for methods of validation and criteria of acceptance for the following programs: - Personnel Practices - Personnel Processing Practices - Premises and Equipment Maintenance - Control of Physical Contaminants - Supplier Approval - Transport and Delivery - Waste management Thank you Eggtech Roy Costa 13.07. Blacklist 2011 Scripts Pdf Files. 11 12:55.
Under Preventive Controls for Human Food, FDA introduced the concept of PCQI. PCQI ( Preventive Controls Qualified Individual) is required to perform certain activities of ensuring and validating if a food manufacturing facility complies with the applicable rules. To become a PCQI, one should be a qualified individual who has “successfully completed training in the development and application of risk-based preventive controls.” Responsibilities of a PCQI include overseeing or performing 1) preparation of the Food Safety Plan, 2) validation of the preventive controls, 3) records review, 4) reanalysis of the Food Safety Plan and other activities as appropriate to the food. Designed for the Preventive Controls Qualified Individual (PCQI), this training course will be offered based on the standardized curriculum designed by FSPCA and recognized by FDA.
At the end of this highly interactive training, you will be able to learn the new FDA requirements for development and application of hazard analysis and risk-based preventive controls. This course is developed by the FSPCA (Food Safety Preventive Controls Alliance) and upon successful completion the Certificate of Training is issued electronically by FSPCA, AFDO(Association of Food and Drug Officials) and IFSH (Institute for Food Safety and Health). The agenda is intended to be covered in a 2.5 day (20 hours) course, including frequent opportunities for review and classroom exercises designed to provide learning opportunities for understanding Preventive Controls for Human Food regulation requirements. The time allotted to each section will vary based on the audience, level of familiarity and experience with Good Manufacturing Practices and risk‐based food safety principles, as well as the food product and processing under consideration. A typical agenda appears below.